Pharmaceutical products have different microbiological considerations and specifications that are determined by the nature of the medicinal product, the method of manufacture, and the intended use of the product. Existing dosage forms are well characterised and understood, and therefore are associated with established and validated specifications, test methods and acceptance criteria. MAP products are a new delivery system and, while learnings from analogous dosage forms are important, they have some unique features that must be considered when assessing the microbiological quality and safety of the product, in terms of the risk of a clinically significant infection.
We therefore brought together a group of stakeholders to identify the pertinent features of MAP products that should be considered when assessing the risk of infection and share their expert opinions and experiences with the scientific community. The published findings of this work can be accessed HERE (Journal of Controlled Release), and will hopefully help to inform microbiological risk assessments for emerging MAP products.
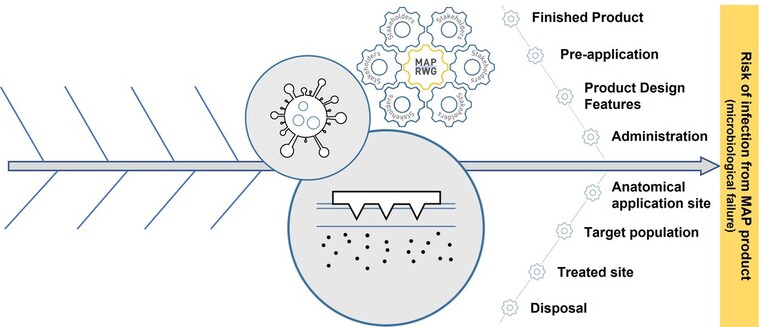
Figure: Graphical Abstract from the MAP-RWG publication entitled "Assessing the risk of a clinically significant infection from a Microneedle Array Patch (MAP) product", published in July 2023 in the Journal of Controlled Release. Click HERE to access this article.
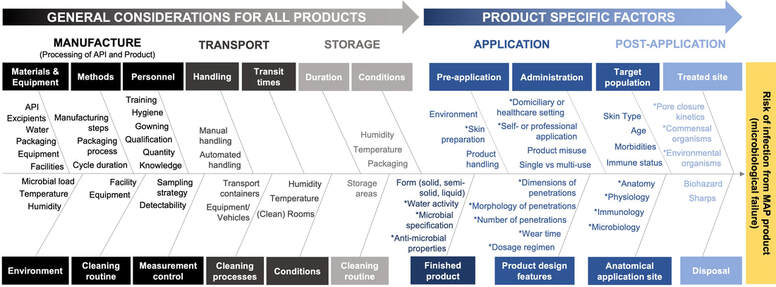
Figure: An Ishikawa diagram, developed by the MAP RWG and members of a MAP-RWG sub-group (MAP Sterility Working Group), to summarise key considerations when assessing the risk of infection from a MAP product. Factors highlighted with an asterisk (*) have been identified as particularly relevant, either because they are exclusive to the dosage form or are particularly pertinent to MAP products. This figure has been reproduced from the MAP-RWG publication entitled "Assessing the risk of a clinically significant infection from a Microneedle Array Patch (MAP) product", published in July 2023 in the Journal of Controlled Release. https://doi.org/10.1016/j.jconrel.2023.07.001
Page updated 15 September 2023